Background: MCL is an aggressive form of NHL. Despite high initial response rates to currently available frontline therapies for newly diagnosed MCL, patients will inevitably experience relapse. Survival outcomes are poor for patients with R/R MCL. Lisocabtagene maraleucel (liso-cel) is an autologous, CD19-directed, 4-1BB CAR T cell product administered at equal target doses of CD8 + and CD4 + CAR + T cells. In the primary analysis of the MCL cohort from the phase 1, seamless design TRANSCEND NHL 001 study (NCT02631044), liso-cel treatment resulted in a rapid, high ORR and high rate of durable CR with low incidences of grade ≥ 3 cytokine release syndrome, neurological events, and infections in heavily pretreated patients with high-risk, aggressive R/R MCL (Wang M, et al. Hematol Oncol 2023). Here, we present patient-reported outcomes (PRO) results from the MCL cohort of TRANSCEND NHL 001.
Methods: Patients had R/R MCL after ≥ 2 lines of prior therapy, including a Bruton tyrosine kinase inhibitor, alkylating agent, and CD20-targeted agent. After leukapheresis and lymphodepleting chemotherapy (LDC), patients received liso-cel. Patients completed the European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire-30 items (EORTC QLQ-C30) and EQ-5D-5L utility index ≤ 7 days before LDC; before infusion on the day of liso-cel infusion (Day 1; baseline); then on Days 29, 60, 90, 180, 270, 365, 545, and 730 posttreatment; and at disease progression/relapse. Based on clinical relevance to MCL, the prespecified primary domains of interest were EORTC QLQ-C30 global health status/quality of life (QOL), physical functioning, fatigue, and pain. PRO analyses were conducted in all MCL patients treated with liso-cel who had an evaluable measure at baseline and at ≥ 1 postbaseline visit. The least squares (LS) mean change from baseline was estimated from a linear mixed-effects model for repeated measures at each time point above. Published minimally important difference thresholds (Cocks K, et al. Eur J Cancer 2012; Pickard AS, et al. Health Qual Life Outcomes 2007) were used to interpret whether LS mean changes from baseline were clinically meaningful. Proportions of patients with clinically meaningful change were calculated using published responder definitions of 5-30 points for EORTC QLQ-C30 domains (Cocks K, et al. Qual Life Res 2023), 0.08 for EQ-5D-5L utility index (Hernández-Alava M, et al. Pharmacoeconomics 2023), and 7 for EQ-5D-5L visual analog scale (EQ-VAS) (Pickard AS, et al. Health Qual Life Outcomes 2007).
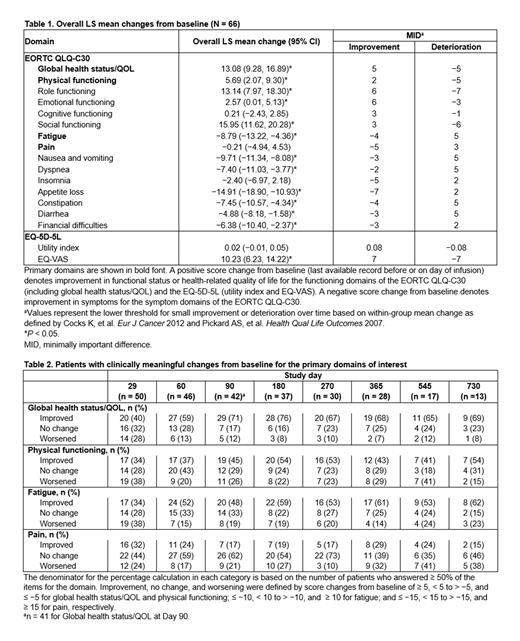
Results: Of 88 patients who received liso-cel, 66 were included in the PRO analyses (median age, 68.5 years [range, 36-86]; 76% male; 89% White; 94% not Hispanic or Latino). Baseline and clinical characteristics were comparable with those of the total liso-cel-treated population, including baseline ECOG PS scores and best response to any prior therapy. Baseline PRO scores for most primary domains were comparable with scores from a US general population norm; other domains fluctuated. Questionnaire completion rates ranged from 55% to 76% from Day 29 to Day 365 postinfusion. Overall LS mean changes from baseline showed significant and meaningful improvements for all primary domains except for pain, as well as for most other PRO domains. Although not showing meaningful improvement, pain scores remained largely unchanged through last assessment (Table 1). Across visits, most patients (60%-100%) experienced meaningful improvement or no meaningful change for all domains (primary domains shown in Table 2). More than 50% of patients achieved meaningful improvement in global health status/QOL, fatigue, social functioning, and EQ-VAS across visits after Day 29. In analyses evaluating the subset of patients in the MCL cohort with secondary CNS lymphoma (n = 5), improvements from baseline exceeded those of the non-secondary CNS population.
Conclusions: Patients with R/R MCL who were treated with liso-cel in the MCL cohort of TRANSCEND NHL 001 showed meaningful improvements across relevant aspects of health-related QOL, including symptoms and functioning. Clinically meaningful improvements were observed for all primary domains except for pain, and for many of the other PRO domains. These findings complement the clinical efficacy and safety data from the primary analysis of the MCL cohort of TRANSCEND NHL 001, further supporting the benefit of liso-cel in patients with R/R MCL.
Disclosures
Wang:Janssen: Consultancy, Honoraria, Research Funding; Kite, a Gilead Company: Consultancy, Honoraria, Other: Travel, Research Funding; Pharmacyclics: Consultancy, Honoraria, Research Funding; VelosBio: Consultancy, Research Funding; Pepromene Bio: Consultancy; Parexel: Consultancy; Oncternal: Consultancy, Research Funding; Milken Institute: Consultancy; Miltenyi Biomedicine: Consultancy; Celgene: Other: Travel, Research Funding; Juno Therapeutics: Research Funding; Genentech: Consultancy, Research Funding; Dava Oncology: Honoraria, Other: Travel; CAHON: Honoraria; Bantam Pharmaceutical: Honoraria; Leukemia & Lymphoma Society: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Eli Lilly and Company: Consultancy, Research Funding; Merck: Consultancy, Honoraria; Nurix: Honoraria; Meeting Minds Experts: Honoraria; NIH: Honoraria; Medscape: Honoraria; i3Health: Honoraria; IDEOlogy Health: Honoraria; Oncology Specialty Group: Honoraria; Genmab: Honoraria, Research Funding; Eastern Virginia Medical School: Honoraria; OncLive: Honoraria; Physicians Education Resources (PER): Honoraria, Other: Travel; Practice Point Communications (PPC): Honoraria; Scripps: Honoraria; Studio ER Congressi: Honoraria; WebMD: Honoraria; AbbVie: Consultancy, Honoraria; Acerta Pharma: Consultancy, Honoraria, Research Funding; ADC Therapeutics America: Consultancy; AstraZeneca: Consultancy, Honoraria, Other: Travel, Research Funding; Amphista Therapeutics Limited: Consultancy; BeiGene: Consultancy, Honoraria, Research Funding; Be Biopharma: Consultancy; Bristol Myers Squibb: Consultancy, Honoraria; BioInvent: Consultancy, Honoraria, Research Funding; Deciphera: Consultancy; MJH Life Sciences: Honoraria; MD Education: Honoraria; Moffit Cancer Center: Honoraria; DTRM Biopharma (Cayman) Limited: Consultancy; Genentech: Consultancy, Research Funding; InnoCare: Consultancy, Research Funding; Loxo Oncology: Consultancy, Research Funding; Molecular Templates: Research Funding; Vincerx: Research Funding; Anticancer Association: Honoraria; BGICS: Honoraria; Clinical Care Options: Honoraria; Epizyme: Consultancy, Honoraria; Hebei Cancer Prevention Federation: Honoraria; Imedex: Honoraria; TS Oncology: Honoraria; Mumbai Hematology Group: Honoraria; OMI: Honoraria; Pharmacyclics: Honoraria; Physicians Education Resources: Honoraria; Practice Point Communications: Honoraria; CSTone: Consultancy. Gordon:Ono Pharmaceuticals: Consultancy; Bristol Meyers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees; Zylem Biosciences: Other: co-founder; Kite Pharmaceuticals: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Other: data and safety monitoring board ; nanoparticles: Patents & Royalties: nanoparticles for cancer therapy (HDL NP As Inducers of Ferroptosis in Cancer, PCT/US2020/051549; Nanostructures: Patents & Royalties: Nanostructures for Treating Cancer and Other Conditions, PCT/US2013/027431). Hirayama:Novartis: Honoraria; Nektar Therapeutics: Honoraria, Research Funding; Bristol Myers Squibb: Honoraria, Research Funding; Juno Therapeutics, a Bristol Myers Squibb Company: Research Funding. Lunning:Sanofi: Consultancy, Honoraria; Regeneron: Consultancy, Honoraria; Pharmacyclics: Consultancy, Honoraria; Nurix: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; Morphosys: Consultancy, Honoraria; Miltenyi: Consultancy, Honoraria; Loxo: Consultancy, Honoraria; Kite: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; Ipsen: Consultancy, Honoraria; InstilBio: Consultancy, Honoraria; GenMab: Consultancy, Honoraria; Genentech: Consultancy, Honoraria; Fate Therapeutics: Consultancy, Honoraria; EUSA: Consultancy, Honoraria; Daiichi Sankyo: Consultancy, Honoraria; CRISPR: Consultancy, Honoraria; Caribou: Consultancy, Honoraria; BMS: Consultancy, Honoraria, Research Funding; Astellas: Consultancy, Honoraria; Astra Zeneca: Consultancy, Honoraria; ADC Therapeutics: Consultancy, Honoraria; Acrotech: Consultancy, Honoraria; AbbVie: Consultancy, Honoraria; SeaGen: Consultancy, Honoraria; Takeda: Consultancy, Honoraria; TG Therapeutics: Consultancy, Honoraria; Curis: Research Funding. Shi:Pharmaceutical Product Development Inc.: Current Employment; Thermo Fisher: Current holder of stock options in a privately-held company; Evidera Inc.: Current Employment. Guo:Evidera Inc.: Current Employment; Bristol Myers Squibb: Consultancy. Kostic:Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company. Eliason:Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company; GlaxoSmith-Kline: Ended employment in the past 24 months. Kumar:Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company. Palomba:Thymofox: Honoraria; Ceramedix: Honoraria; Juno: Honoraria, Patents & Royalties; Kite: Honoraria; MustangBio: Honoraria; Pluto Immunotherapeutics: Honoraria; Rheos: Honoraria; Smart Immune: Honoraria; Seres Therapeutics: Honoraria, Patents & Royalties; GarudaTherapeutics: Honoraria; Cellectar: Honoraria; Novartis: Honoraria; BMS: Honoraria; Synthekine: Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal